UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a‑16 OR 15d‑16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF JANUARY 2022
COMMISSION FILE NUMBER 001-39081
BioNTech SE
(Translation of registrant’s name into English)
(Translation of registrant’s name into English)
An der Goldgrube 12
D-55131 Mainz
Germany
+49 6131-9084-0
(Address of principal executive offices)
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20‑F or Form 40‑F: Form 20‑F ☒ Form 40‑F ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(7): ☐
DOCUMENTS INCLUDED AS PART OF THIS FORM 6-K
On January 11, 2022, BioNTech SE (the “Company”) CEO and co-founder Ugur Sahin presented at the JP Morgan Healthcare Conference 2022. The presentation is attached hereto as Exhibit 99.1.
SIGNATURE
Pursuant to the requirements of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| BioNTech SE | ||||||||
| By: | /s/ Dr. Sierk Poetting | |||||||
| Name: Dr. Sierk Poetting | ||||||||
| Title: Chief Operating Officer | ||||||||
Date: January 11, 2022
EXHIBIT INDEX
| Exhibit | Description of Exhibit | ||||
| 99.1 | |||||

1 January 11th 2022 J.P. MORGAN HEALTHCARE CONFERENCE Exhibit 99.1

2 Titelmasterformat durch Klicken bearbeiten This slide presentation includes forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech's expected revenues and net profit related to sales of BioNTech's COVID-19 vaccine, referred to as COMIRNATY® where approved for use under full or conditional marketing authorization, in territories controlled by BioNTech's collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech's partners; BioNTech's pricing and coverage negotiations with governmental authorities, private health insurers and other third-party payors after BioNTech's initial sales to national governments; the extent to which initial or booster doses of a COVID-19 vaccine continue to be necessary in the future; competition from other COVID-19 vaccines or related to BioNTech's other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the rate and degree of market acceptance of BioNTech's COVID-19 vaccine and, if approved, BioNTech's investigational medicines; the collaboration between BioNTech and Pfizer to develop a COVID-19 vaccine (including aa potential booster dose of BNT162b2 and/or a potential booster dose of a variation of BNT162b2 having a modified mRNA sequence); the ability of BNT162b2 to prevent COVID-19 caused by emerging virus variants; BioNTech's ability to identify research opportunities and discover and develop investigational medicines; the initiation, timing, progress, results, and cost of BioNTech's research and development programs and BioNTech's current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and BioNTech's research and development programs; the timing of and BioNTech's ability to obtain and maintain regulatory approval for BioNTech's product candidates; the ability and willingness of BioNTech's third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; the impact of the COVID-19 pandemic on BioNTech's development programs, supply chain, collaborators and financial performance; unforeseen safety issues and claims for personal injury or death arising from the use of BioNTech's COVID-19 vaccine and other products and product candidates developed or manufactured by us; BioNTech's ability to progress BioNTech's Malaria, Tuberculosis and HIV programs, including timing for selecting clinical candidates for these programs and the commencement of a clinical trial, as well as any data readouts; the nature of the collaboration with the African Union and the Africa CDC; the nature and duration of support from WHO, the European Commission and other organizations with establishing infrastructure; the development of sustainable vaccine production and supply solutions on the African continent and the nature and feasibility of these solutions; BioNTech's estimates of research and development revenues, commercial revenues, cost of sales, research and development expenses, sales and marketing expenses, general and administrative expenses, capital expenditures, income taxes, shares outstanding; BioNTech's ability and that of BioNTech's collaborators to commercialize and market BioNTech's product candidates, if approved, including BioNTech's COVID-19 vaccine; BioNTech's ability to manage BioNTech's development and expansion; regulatory developments in the United States and foreign countries; BioNTech's ability to effectively scale BioNTech's production capabilities and manufacture BioNTech's products, including BioNTech's target COVID-19 vaccine production levels, and BioNTech's product candidates; and other factors not known to BioNTech at this time. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially from those expressed or implied by these forward- looking statements. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech's quarterly report for the three and nine months ended September 30, 2021 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at https://www.sec.gov/. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any for-ward-looking statements contained in this quarterly report in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof.

Titelmasterformat durch Klicken bearbeiten Safety Information COMIRNATY® ▼(COVID-19 mRNA Vaccine) has been granted conditional marketing authorisation by the European Medicines Agency to prevent coronavirus disease 2019 (COVID-19) in people from 5 years of age and older. EMA’s human medicines committee (CHMP) has completed its rigorous evaluation of COMIRNATY ®, concluding by consensus that sufficiently robust data on the quality, safety and efficacy of the vaccine are now available. IMPORTANT SAFETY INFORMATION • Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with a known hypersensitivity to the active substance or to any of the excipients listed • Events of anaphylaxis have been reported. Appropriate medical treatment and supervision should always be readily available in case of an anaphylactic reaction following the administration of the vaccine • There is an increased risk of myocarditis and pericarditis following vaccination with Comirnaty. These conditions can develop within just a few days after vaccination and have primarily occurred within 14 days. They have been observed more often after the second vaccination, and more often in younger males. Healthcare professionals should be alert to the signs and symptoms of myocarditis and pericarditis • Anxiety-related reactions, including vasovagal reactions (syncope), hyperventilation or stress‐related reactions (e.g., dizziness, palpitations, increases in heart rate, alterations in blood pressure, tingling sensations and sweating) may occur in association with the vaccination process itself. It is important that precautions are in place to avoid injury from fainting • Vaccination should be postponed in individuals suffering from acute severe febrile illness or acute infection. The presence of a minor infection and/or low-grade fever should not delay vaccination. • As with other intramuscular injections, the vaccine should be given with caution in individuals receiving anticoagulant therapy or those with thrombocytopenia or any coagulation disorder (such as haemophilia) because bleeding or bruising may occur following an intramuscular administration in these individuals • The efficacy, safety and immunogenicity of the vaccine has not been assessed in immunocompromised individuals, including those receiving immunosuppressant therapy. The efficacy of COMIRNATY® may be lower in immunosuppressed individuals. • The duration of protection afforded by the vaccine is unknown as it is still being determined by ongoing clinical trials. • As with any vaccine, vaccination with COMIRNATY ® may not protect all vaccine recipients. Individuals may not be fully protected until 7 days after their second dose of vaccine. • Comirnaty ® has no or negligible influence on the ability to drive and use machines. However, some of side effects mentioned below, may temporarily affect the ability to drive or use machines. • The overall safety profile of Comirnaty® in participants 5 to 15 years of age was similar to that seen in participants 16 years of age and older. • The most frequent adverse reactions in children 5 to 11 years of age were injection site pain (>80%), fatigue (>50%), headache (>30%), injection site redness and swelling (>20%), myalgia and chills (>10%). • The most frequent adverse reactions in adolescents 12 to 15 years of age that received 2 doses were injection site pain (> 90%), fatigue and headache (> 70%), myalgia and chills (> 40%), arthralgia and pyrexia (> 20%). • In clinical studies, the most frequent adverse reactions in participants 16 years of age and older that received 2 doses were injection site pain (> 80%), fatigue (> 60%), headache (> 50%), myalgia (> 40%), chills (> 30%), arthralgia (> 20%), pyrexia and injection site swelling (> 10%) and were usually mild or moderate in intensity and resolved within a few days after vaccination. A slightly lower frequency of reactogenicity events was associated with greater age. • In clinical trials, the most frequent adverse reactions in participants 18 to 55 years of age who received a booster were injection site pain (> 80%), fatigue (> 60%), headache (> 40%), myalgia (> 30%), chills and arthralgia (> 20%). • There is limited experience with use of COMIRNATY® in pregnant women. Administration of COMIRNATY® in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus. • It is unknown whether COMIRNATY® is excreted in human milk. • Interactions with other medicinal products or concomitant administration of COMIRNATY® with other vaccines has not been studied. • For complete information on the safety of COMIRNATY® always make reference to the approved Summary of Product Characteristics and Package Leaflet available in all the languages of the European Union on the EMA website. The black equilateral triangle denotes that additional monitoring is required to capture any adverse reactions. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. Side effects can be reported to EudraVigilance [http://www.adrreports.eu/] or directly to BioNTech using email medinfo@biontech.de, telephone +49 6131 9084 0, or our website https://medicalinformation.biontech.de/ 3

Titelmasterformat durch Klicken bearbeiten Safety Information AUTHORIZED USE IN THE U.S. COMIRNATY® (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. It is also authorized under EUA to provide a 2-dose primary series to individuals 5 years of age and older, a third primary series dose to individuals 12 years of age and older who have been determined to have certain kinds of immunocompromise, a single booster dose to individuals 16 years of age and older who have completed a primary series with Pfizer-BioNTech COVID-19 Vaccine or COMIRNATY®, a single booster dose to individuals 18 years of age and older who have completed primary vaccination with a different authorized COVID-19 vaccine. The booster schedule is based on the labeling information of the vaccine used for the primary series. IMPORTANT SAFETY INFORMATION Individuals should not get the vaccine if they: • had a severe allergic reaction after a previous dose of this vaccine • had a severe allergic reaction to any ingredient of this vaccine Individuals should tell the vaccination provider about all of their medical conditions, including if they: • have any allergies • have had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart) • have a fever • have a bleeding disorder or are on a blood thinner • are immunocompromised or are on a medicine that affects the immune system • are pregnant, plan to become pregnant, or are breastfeeding • have received another COVID-19 vaccine • have ever fainted in association with an injection The vaccine may not protect everyone. Side effects reported with the vaccine include: • There is a remote chance that the vaccine could cause a severe allergic reaction o A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose of the vaccine. For this reason, vaccination providers may ask individuals to stay at the place where they received the vaccine for monitoring after vaccination o Signs of a severe allergic reaction can include difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body, dizziness, and weakness o If an individual experiences a severe allergic reaction, they should call 9-1-1 or go to the nearest hospital • Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received the vaccine. In most of these people, symptoms began within a few days following receipt of the second dose of the vaccine. The chance of having this occur is very low. Individuals should seek medical attention right away if they have any of the following symptoms after receiving the vaccine: o chest pain o shortness of breath o feelings of having a fast-beating, fluttering, or pounding heart • Additional side effects that have been reported with the vaccine include: o severe allergic reactions; non-severe allergic reactions such as rash, itching, hives, or swelling of the face; myocarditis (inflammation of the heart muscle); pericarditis (inflammation of the lining outside the heart); injection site pain; tiredness; headache; muscle pain; chills; joint pain; fever; injection site swelling; injection site redness; nausea; feeling unwell; swollen lymph nodes (lymphadenopathy); decreased appetite; diarrhea; vomiting; arm pain; fainting in association with injection of the vaccine • These may not be all the possible side effects of the vaccine. Serious and unexpected side effects may occur. The possible side effects of the vaccine are still being studied in clinical trials. Call the vaccination provider or healthcare provider about bothersome side effects or side effects that do not go away Data on administration of this vaccine at the same time as other vaccines have not yet been submitted to FDA. Individuals considering receiving this vaccine with other vaccines, should discuss their options with their healthcare provider. Patients should always ask their healthcare providers for medical advice about adverse events. Individuals are encouraged to report negative side effects of vaccines to the US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). Visit https://www.vaers.hhs.gov or call 1-800-822-7967. In addition, side effects can be reported to Pfizer Inc. at www.pfizersafetyreporting.com or by calling 1-800-438-1985. 4

5 Harnessing the power of the immune system to fight human diseases 5

6 BioNTech 2021 Highlights | A Year of Historic Impact ~ 2.6 bn doses shipped2, 3 First ever approved mRNA therapy1 > 1 bn individuals vaccinated4 Trillions of dollars of global economic impact5 > 160 countries / regions reached 1Approved for emergency use/temporary supply or Conditional Marketing Authorization in more than 90 countries worldwide including the U.S. and EU, December 2021 2As of mid December 2021 3. Manufactured approximately 3 bn doses in 2021 4. Efficacy against symptomatic infection, Polack FP, et al. NEJM 2020, 383:2603-2615; 4Eric C. Schneider et al., The U.S. COVID-19 Vaccination Program at One Year: How Many Deaths and Hospitalizations Were Averted? (Commonwealth Fund, December 2021); European Centre for Disease Prevention and Control; 5Statista Millions of cases of severe illness or death likely averted4 BNT162b2 ~ 1 bn to low- and middle-income countries2 Fastest pharma product development and launch 6

7 BioNTech 2021 Highlights (cont.) | A Year of Historic Impact Pipeline expansion Global presence Production capacity Commercial infrastructure R&D expansion Financial performance 9 oncology clinical trial starts, including 4 Phase 2 trials and 5 FIH studies Acquired and integrated new site in Gaithersburg, US; Established offices in Singapore, China and Turkey Commercial scale mRNA production and addition of US cell therapy manufacturing facility Deployed commercial team in Germany Increased R&D team by >40% to more than 850 professionals FIH, first-in-human 7

8 BioNTech Commercial Revenues (M€)2 Enabled by early scale up of production capacity: ~3 bn doses manufactured in 2021 Estimated COMIRNATY market share 74% in U.S. and 80% in Europe1 Outlook for COVID-19 vaccine revenues booked by BioNTech FY 2021 guidance: €16-17 bn FY 2022 estimate: €13-17 bn 1As of December 2021; 2Represents an estimated figure based on preliminary data shared between Pfizer and BioNTech. Changes in share of the collaboration partner’s gross profit will be recognized prospectively. Graphic is for illustration only 2,028 280 5,281 6,040 Q3 2021Q2 2021Q1 2021Q4 2020 Strong Financial Performance Historic Chance to Accelerate our Vision Through Reinvestment in the Company

9 BioNTech Today | A 21st Century Immunotherapy Powerhouse Multi-Platform Strategy • Technology agnostic innovation engine • Entering a new era of mRNA technology & synthetic biologyFully Integrated Biotechnology Company • Digitalization & automation poised to transform R&D • 4 GMP manufacturing facilities • Commercial capability built in Germany • 3,000+ team members from 60+ countries Diversified Product Pipeline • 1 approved vaccine • 16+ clinical stage product candidates • 30+ programs Our Approach to Global Social Responsibility • Focus on high medical needs • Democratize access to novel medicines

1010

11 mRNA Vaccines Targeted Antibodies Small Molecule Immunomodulators • Targeting immune checkpoint molecules • Engineered bispecific antibodies • Enginereed MOAs Cell & Gene Therapies Next Generation Immunomodulators Ribologicals • Against highly selective cancer cell surface antigens for high precision • Selective TLR 7 antagonism • CAR-T cells • Personalized TCR therapies • Polyspecific T cell therapies • In vivo engineered cell therapies • Individualized cancer vaccines (iNEST) • Off-the-shelf cancer vaccines (FixVac) • Antigen-specific tolerance vaccines • Prophylactic infectious disease vaccines • Antibodies (RiboMabs) • Cytokines (RiboCytokines) mRNA encoded effector molecules Multi-Platform Strategy | Technology Agnostic Innovation Engine Multiple product classes with unique combination potential MOA, mechanism of action
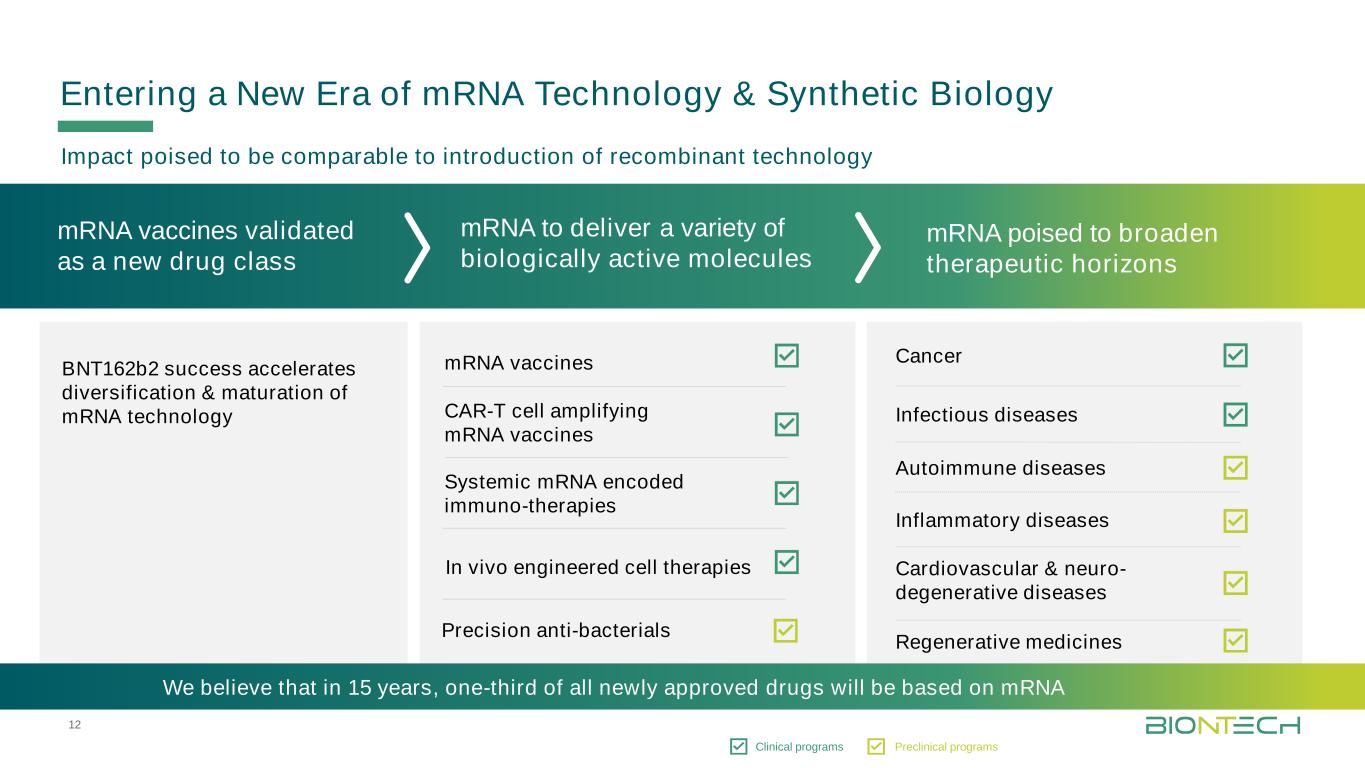
12 Titelmasterformat durch Klicken bearbeiten In vivo engineered cell therapies Precision anti-bacterials CAR-T cell amplifying mRNA vaccines Systemic mRNA encoded immuno-therapies mRNA vaccines Autoimmune diseases mRNA vaccines validated as a new drug class Entering a New Era of mRNA Technology & Synthetic Biology BNT162b2 success accelerates diversification & maturation of mRNA technology mRNA to deliver a variety of biologically active molecules mRNA poised to broaden therapeutic horizons • Impact poised to be comparable to introduction of recombinant technology Cancer Cardiovascular & neuro- degenerative diseases Infectious diseases Regenerative medicines Inflammatory diseases Clinical programs Preclinical programs We believe that in 15 years, one-third of all newly approved drugs will be based on mRNA

1313

14 FixVac – 1L Melanoma FixVac – HPV16+ Cancers iNeST2 – 1L Melanoma iNeST2 – Adjuvant CRC Bispecific AB PD-L1x4-1BB3 Phase 1 ongoing Phase 2 ongoing Significant Pipeline Expansion and Maturation in 2022 COVID-19 Vaccine1 Infectious DiseasesImmuno-Oncology New AreasG O A L S 5 mRNA vaccines in human trials Global Leadership 5 randomized phase 2 trials Multiple programs in lead candidate selection 1Partnered with Pfizer; 2Partnered with Genentech; 3Partnered with Genmab in a 50/50 collaboration; 4 Partnered with BMGF Label expansions Pediatric dosage forms Variant adaptations Influenza1 Shingles1 Malaria Tuberculosis4 HSV 2 Cardiovascular diseases Neurodegenerative diseases Autoimmune diseases Multiple new product launches Near- and Mid-term Growth Drivers Long-term Growth Drivers

15 BNT162b2 Poised for Continued Global Impact in 2022 • >3 billion people globally not yet vaccinated1 • > 25% of global population ages 0-142 • First vaccine to launch for children 6 mths – 5 yrs of age pending marketing authorization • > 600 m people already boosted3 • Booster vaccination vital to mitigate impact of Omicron4 • Omicron variant-specific vaccine approach could be ready as early as March 2022, subject to regulatory approval • Rapid vaccine adaption process for future variants, if needed 1WHO & United Nations; 2The World Bank; 3Our World in Data; 4BioNTech Press Conference, December 8, 2021, slide 9; https://investors.biontech.de/static-files/47b4131a-0545-4a0b-a353-49b3a1d01789. Up to 4B doses manufacturing capacity in 2022 Doses for the unvaccinated Ensuring equitable access Adolescent / pediatric doses 3rd Booster Variant-specific vaccine • 2 bn doses pledged to low- and middle-income countries through end of 2022

16 COVID-19: The long road to an endemic disease Protection of Individuals with immune compromises and medical conditions at risk Risk for Long COVID and organ damage associated even with mild / moderate infections2 Waning of protection over time in broader population Risk of MIS-C; incidence of 0.5% ─ 3.1% of all children1 1 Canadian Paediatric Society; 2 Destin Groff et al. JAMA Network.2021 https://jamanetwork.com/searchresults?author=Anna+E.+Ssentongo&q=Anna+E.+Ssentongo COVID-19 will remain a high- medical need global challenge New variants rapidly spreading at global level Need for sterilizing immunity in frontline workers Significant impact on infrastructure and economy Continued need for regular booster vaccinations and pediatric vaccinations Huge global SARS-CoV-2 virus reservoir drives fast evolution of the virus

17 Early Computational Detection of High Risk SARS-CoV-2 Variants* Early Warning System (EWS) combines Spike protein structural modeling with artificial intelligence (AI) to detect and monitor high risk SARS-CoV-2 variants a lt e re d a n ti b o d y e p it o p e s EWS identifies and scores >90% of new variants on average two months prior to their official designation by WHO *Artificial intelligence Collaboration of BioNTech and InstaDeep https://www.biorxiv.org/content/10.1101/2021.12.24.474095v1

18 Titelmasterformat durch Klicken bearbeiten Infectious Disease Product Strategy Rooted in Global Social Responsibility mRNA-based vaccines and therapeutics Malaria: 229 million cases and 409,000 deaths annually1 Tuberculosis*: 10 million people contracted TB in 20192 HIV*: 37.7 million people living with HIV, two-thirds in WHO African region3 Advancing programs to combat major health burdens Democratizing global access to mRNA medicines Ensuring equitable COVID-19 vaccine access to LMICs4 Expanding COVID-19 manufacturing network to Africa and South America Construction of state-of-the-art mRNA manufacturing sites in Africa and Asia in mid-2022 to establish sustainable local supply 1 World Health Organization https://www.who.int/news-room/fact-sheets/detail/malaria 2 World Health Oganization https://www.who.int/news-room/fact-sheets/detail/tuberculosis 3 World Health Organization https://www.who.int/data/gho/data/themes/hiv-aids 4Low- and low-middle income countries mRNA Vaccines | Ribologicals | Synthetic Lysins * Collaboration with Bill & Melinda Gates Foundation.

19 Titelmasterformat durch Klicken bearbeiten 5 mRNA Vaccines in Human Trials in 2022 3 mRNA vaccines partnered w/Pfizer 10+ other infectious disease programs Platform Pre-clinical Phase 1 Phase 2 Commercial Economics COVID-19 Vaccine Cost / Gross profit split Influenza Milestones and royalties Shingles Cost / Gross profit split Malaria Tuberculosis1 HSV 2 HIV1 Additional mRNA vaccine programs2 Precision antibacterials 1 Collaboration with Bill & Melinda Gates Foundation. BioNTech holds worldwide distribution rights except developing countries where BMG holds distribution rights; 2 University of Pennsylvania collaboration Expected Phase 1 trial initiation in 2022 Worldwide rights to BioNTech

20 Oncology Pipeline Expected to Significantly Expand Drug class Platform Product candidate Indication (targets) Pre-clinical Phase 1 Phase 2 Phase 3 Partner mRNA FixVac* (fixed combination of shared cancer antigens) BNT111 Advanced melanoma (Adjuvant & Metastatic) BNT112 Prostate cancer BNT113 HPV16+ head and neck cancer BNT1151 Ovarian cancer1 BNT116 NSCLC iNeST (patient specific cancer antigen therapy) Autogene cevumeran (BNT122) 1L melanoma GenentechAdjuvant colorectal cancer Solid tumors Intratumoral Immunotherapy SAR441000 (BNT131) Solid tumors (IL-12sc, IL15-sushi, GM-CSF, IFNα) Sanofi RiboMabs* (mRNA-encoded antibodies) BNT141 Multiple solid tumors (CLDN18.2) BNT142 Multiple solid tumors (CD3+CLDN6) RiboCytokines* (mRNA-encoded cytokines) BNT151 Multiple solid tumors (optimized IL-2) BNT152, BNT153 Multiple solid tumors (IL-7, IL-2) Cell Therapies CAR-T Cells* BNT211 Multiple solid tumors (CLDN6) BNT212 Pancreatic, other cancers (CLDN18.2) Neoantigen-based T cell therapy* BNT221 (NEO-PTC-01) Multiple solid tumors TCRs* To be selected All tumors Antibodies Next-Gen CP Immunomodulators GEN1046 (BNT311) Multiple solid tumors (PD-L1x4-1BB) Genmab GEN1042 (BNT312) Multiple solid tumors (CD40x4-1BB) Targeted Cancer Antibodies BNT321 (MVT-5873) Pancreatic cancer (sLea) SMIM Toll-Like Receptor Binding BNT411 Solid tumors (TLR7) 1 investigator-initiated Phase 1 trial; CP; Checkpoint inhibitor; SMIM, Small molecule immunomodulators; * Fully-owned rights 20

Titelmasterformat durch Klicken bearbeitenBNT211: Phase 1/2 Trial Evaluating Next Generation CAR-T Targeting Claudin-6 with CARVac in Solid Tumors CLDN6, Claudin-6; CAR-T cells, chimeric antigen receptor engineered T cells; scFv, single chain variable fragment; RP2D, recommended Phase 2 dose; NOS, not otherwise specified; Reinhard K, et al. Science 2020; 367:446-453 CAR-T cell therapy + RNA Vaccine to amplify CAR-T cell (CARVac) in vivo • 2nd generation CAR directed against CLDN6, a cancer specific carcino-embryonic antigen • CLDN6 is expressed in multiple solid cancers with high medical need • CARVac drives in vivo expansion, persistence and efficacy of CAR-T BNT211 CAR Structure Part 2 CLDN6 CAR-T dose escalation + CLDN6 CARVac Part 1 CLDN6 CAR-T dose escalation (3 dose levels) Patients with CLDN6+ relapsed/ refractory solid tumors (up to 36 patients) Part 3 Expansion Cohorts • Ovarian Cancer • Testicular Cancer • Endometrial Cancer • Lung Cancer • Gastric Cancer • Tumors NOS High CLDN6 expression RP2D CLDN6 not present in healthy tissues CLDN6 expressed in multiple cancers 21

Titelmasterformat durch Klicken bearbeitenESMO-IO 2021/BNT211 Phase 1/2: CAR-T Engraftment and Tolerable Safety Profile with CLDN6 CAR-T without (Part 1) and with (Part 2) CARVac Data cutoff = NOV 18, 2021; CRS, cytokine release syndrome; DL, dose level; DLT, dose-limiting toxicity; Haanen J., et al. Oral presentation at the ESMO Immuno-Oncology Congress, December 08–14, 2021; Haanen J. et al. Anals fo Concology (2021) 32 (suppl._7): S1392-S1397 Cohort/Patient Characteristics Part 1 DL1 (n=3) Part 2 DL1 (n=3) Part 1 DL2 (n=6) Part 2 DL2 w/ LD (n=2) Part 2 DL2 w/o LD (n=1) All patients (n=15) Median (range) age, years 33 (25-68) 41 (27-56) 56 (35-66) 53.5 (46-61) 56 54 (25-68) Cancer type, n Testicular Ovarian Endometrial Fallopian tube Sarcoma Gastric 1 1 0 0 1 0 3 0 0 0 0 0 2 1 1 1 0 1 0 2 0 0 0 0 1 0 0 0 0 0 7 4 1 1 1 1 Median (range) CLDN6 II/III+ cells, % 60 (60-80) 90 (90-95) 82.5 (50-90) 95 (90-100) 85 85 (50-100) Median (range) of prior treatment lines 4 (3-5) 4 (3-4) 5 (2-11) 6 (5-7) 4 4 (2-11) • CLDN6 CAR-T cells alone or combined with CARVac well tolerated at the dose levels evaluated to date with only 1 DLT observed • CRS was seen in 1 patient at DL1 + CARVac and 6 patients at DL2, and was manageable by administration of tocilizumab • Robust engraftment of CAR-T cells resulting in a total amount of around 109 achieved in most patients and seems predictive for clinical activity • 9 of 10 patients evaluable for efficacy assessment showed initial disease control including 4 PRs (3 in testicular cancer patients with recent relapse after HDCT/ASCT ) Safety Efficacy 22

BNT211 Phase 1/2: First Indications of Clinical Activity - 4 PR, 4 SD+, 1 SD at 6 Weeks Post Infusion (ORR 4/10, DCR 9/10) Data cutoff = NOV 18, 2021. Haanen J., et al. Oral presentation at the ESMO Immuno-Oncology Congress, December 08–14, 2021; Haanen J. et al., Anals fo Concology (2021) 32 (suppl._7): S1392-S1397 ASCT, autologous stem cell transplantation; DCR, disease control rate; EoT, end of trial (due to PD); HDCT, high-dose chemotherapy; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease, SD+, SD with shrinkage of target lesions; wks, weeks; 1w/o lymphodepletion. ACT 6 weeks 12 weeks 18 weeks 24 weeks 30 weeks 36 weeks 42 weeks 48 weeks Part1 DL1 Part2 DL1 Part1 DL2 Part2 DL2 PR SD+ PD CR EoT Redosing SD No. 1 No. 2 No. 3 Patient No. 1 No. 2 No. 3 No. 1 No. 2 No. 3 No. 4 No. 5 No. 6 No. 1 No. 2 No. 11 23 Part 2 DL1 Patient #3

24

Outlook 2022 and Beyond Further COVID-19 vaccine launches of new formulation, pediatric dosage form, and potentially variant adapted vaccine Accelerate late- stage Oncology programs towards the market and expand earlier stage pipeline Ramp up R&D investment and make strategic investments in cutting edge digital technologies and capabilities Pursue complementary acquisitions of synergistic technologies, infrastructure, and product candidates Expand global organization in Europe, the U.S., Asia, and Africa and deploy pandemic response capability Bring long-term value to patients, shareholders, and society Once in a generation opportunity to transform medicine 25

26
